Stress Granule Protein Entwines and Misfolds Tau
Quick Links
By temporarily sequestering mRNA, stress granules provide a reprieve for cells struggling momentarily with the burden of protein synthesis. Alas, if the granules persist, they may be more trouble than they are worth. In the May 17 Cell Reports, researchers led by Benjamin Wolozin at Boston University report that the RNA-binding protein TIA1, a principal component of stress granules, promotes pathological misfolding of tau. In turn, tau seems to affect the trafficking of TIA1 and other stress granule proteins. These interactions occur primarily in the cell’s soma and dendrites, where tau mysteriously accumulates in neurodegenerative diseases such as Alzheimer’s. "Our findings suggest that tau shifts from axons to the somatodendritic compartment to facilitate stress granule formation,” Wolozin told Alzforum.
“I think this mechanism is very elegant, and it shows how TIA1 regulation could play a crucial role in mitigating tau toxicity,” Chad Dickey, University of South Florida, wrote to Alzforum. TIA1 stands for T cell intracellular antigen 1.
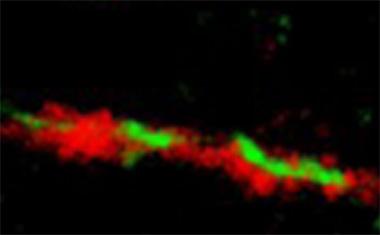
Fatal Attraction.
Super resolution microscopy shows TIA1 (red) binding misfolded tau (green). [Courtesy of the Ben Wolozin lab.]
Stress granules became a focus of neurodegenerative disease research when scientists found that they accumulate in brains affected by amyotrophic lateral sclerosis, frontotemporal dementia, and even AD. Researchers have studied them because they are hotbeds for aggregates of TDP-43 and other RNA-binding proteins that accumulate in damaged neurons (see Jul 2010 conference news; Dec 2013 news; Nov 2015 conference news).
Previously, Wolozin’s group reported that tau, especially mutant disease-associated forms, promotes stress granule formation (see Jun 2012 news). Now they report the inverse, that stress granule components exacerbate tau toxicity.
To tease out interactions between tau and stress granules components, joint first authors Tara Vanderweyde, Daniel Apicco, and Katherine Youmans-Kidder focused on TIA1 because it is a core building block of stress granules. They found that in HT22 mouse hippocampal cells, TIA1 caused wild-type tau to misfold and aggregate into granules that persisted for hours. The effect was more pronounced for the P301L variant of tau that causes frontotemporal dementia. Knocking down TIA1 in hippocampal cells using short hairpin RNAs prevented both wild-type and mutant tau from misfolding (see figure below). Removing TIA1 also protected against tau-driven neurodegeneration in cells. When the researchers transfected wild-type hippocampal neurons with P301L tau, the cells retracted their dendrites less and activated cell death pathways. Both were suppressed in TIA1-negative cells.
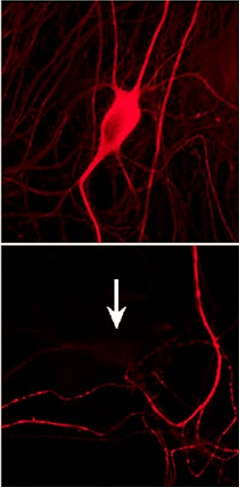
Taming tau.
Researchers eliminated misfolded tau (top) by knocking down TIA1 (bottom). [Image courtesy Wolozin lab, Cell Reports, Creative Commons.]
Inhibitors of PKR and PERK—kinases that promote stress granules—also prevent tau misfolding, the scientists report. On the other hand, treatments that ramp up stress granule formation, such as the protein synthesis inhibitor puromycin, increase the number of tau granules. Overall, the findings suggest that stress granules may be intimately tied up with tau toxicity.
Looking at the stress granule relationship another way, the researchers found that tau seems to influence when and where these inclusions appear. For example, they report that while TIA1 resides in the nucleus in tau knockout hippocampal neurons, as it does in most cells, introducing wild-type or P301L tau into those cells shifted TIA1 into the somatodendritic compartment. In fact, tau seemed to slow down both anterograde and retrograde movement of TIA1 granules, but more so the latter. This led to larger and more granules. When the researchers stressed the cells, for example by adding arsenite, a chemical that induces stress granules, the number of stress granules rose further.
To Wolozin, the findings are a clear indication that tau promotes stress granule formation. A look at TIA1 binding partners supports that conclusion, he said. Vanderweyde and colleagues used immunoprecipitation to survey the interactome of RNA-binding proteins in wild-type and tau knockout mouse brain. They were surprised to find that far fewer interactions occurred in the latter. Also, nature of the interactions changed. In normal tau-containing cells, RNA-binding proteins and proteins involved in stress granule formation dominated, but in tau-negative cells, TIA1 IP failed to pull down many of those proteins.
Co-author Dan Apicco also found that some of those binding partners, including EWSR1 and RPL7, accumulate with misfolded tau in Tg4510 transgenic mice. “This data suggests that tau misfolding might be part and parcel of translational regulation during times of cellular stress,” said Wolozin.
He also thinks tau’s involvement in stress granule dynamics might explain why tau has evolved to be so readily phosphorylated. “We all know that tau gets hyperphosphorylated in disease, but we don’t really know why,” he noted. Vanderweyde and colleagues found that phosphomimetic forms of tau readily associate with TIA1, but not phospho-null forms.
Dickey offered a slightly different perspective. “I think we are learning that there are many ways for a neuron to die when it’s exposed to high levels of aggregating tau. Since tau can take on so many structures, these structures have the potential to disrupt a number of pathways that lead to toxicity; mitochondrial pathways, protein quality control pathways, and stress pathways.”
Either way, Wolozin says these results suggest new ways to think about intervening to prevent tau toxicity. “The brute force way would be to knock out TIA1, but there may be better approaches,” he said. He recently started a company called Aquinnah Pharmaceuticals to pursue this idea.—Tom Fagan
References
News Citations
- Honolulu: TDP-43 Gets a Place in the Sun
- FUS RNA Granules Not So Stressed Out?
- Stressed Out: RNA-binding Protein Inhabits Granules in ALS, FTD
- Paper Alert: Stress Granules Get Tangled Up in Tau
Mutations Citations
Research Models Citations
Further Reading
No Available Further Reading
Primary Papers
- Vanderweyde T, Apicco DJ, Youmans-Kidder K, Ash PE, Cook C, Lummertz da Rocha E, Jansen-West K, Frame AA, Citro A, Leszyk JD, Ivanov P, Abisambra JF, Steffen M, Li H, Petrucelli L, Wolozin B. Interaction of tau with the RNA-Binding Protein TIA1 Regulates tau Pathophysiology and Toxicity. Cell Rep. 2016 May 17;15(7):1455-1466. Epub 2016 May 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Edinburgh
This paper by Vanderweyde and colleagues elegantly demonstrates a role for tau in regulating the interactions of the RNA-binding protein TIA1. In cell culture, they demonstrate that interactions between TIA1 and tau influence stress granule formation and tau-associated toxicity. A very well-controlled analysis of the TIA1 interacting proteome in brain tissue from wild-type mice and tau knockout mice indicate that tau is important for the protein interactome network of TIA1. These data are interesting because they implicate RNA-binding proteins in the pathophysiology of tauopathy.
RNA-binding proteins contribute to the pathophysiology of ALS, and these data add to the growing body of evidence that ALS and frontotemporal dementias lie on a disease spectrum. Although tau pathology is observed in some ALS patients, genetically, tau is one of the molecules that has been thought of as “purely” on the FTD side of this spectrum. These data indicate that similar RNA-mediated mechanisms may be at play in degeneration mediated by tau and more classically ALS proteins such as TDP43 and FUS. It will be interesting to see future studies in human tissue and perhaps knock-in or regulatable models to extend and confirm these results.
Make a Comment
To make a comment you must login or register.