Could Kink in Tau Lead to Neurodegeneration?
Quick Links
Traumatic brain injury can cause aggregation and accumulation of tau, leading to a condition called chronic traumatic encephalopathy (CTE). Scientists are unsure whether tau pathology is a cause or simply a consequence of CTE. A new study led by Kun Ping Lu and Xiao Zhen Zhou, Harvard Medical School, Boston, suggests the former. They report that brain trauma causes isomerization of a specific proline in tau, causing a structural twist that opens the protein up to aggressive phosphorylation and then aggregation. What’s more, their study, published July 15 in Nature, hints that an antibody to the toxic isomer could halt pathology early on. Some researchers are skeptical, noting that prior work linking this isomerized form of tau to Alzheimer's disease pathology has not panned out and claiming that this study may be underpowered. Separately, Sam Gandy of Mount Sinai Medical Center, New York, warned against overenthusiasm. "The observations are dramatic and exciting, but will have to be replicated," he told Alzforum.
“Treating TBI in mice with this antibody prevented the development and spread of tauopathy as well as neuronal loss, and preserved brain structure, function, and volume,” Lu told Alzforum. “It could be an early intervention to prevent the long-term consequences of brain injury.”
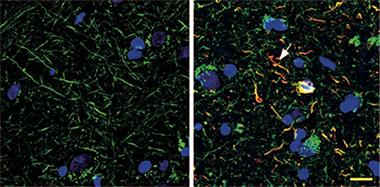
Invasion of the Axons:
Cis p-tau (red) is absent from healthy human brains (left), but pervades the axons (green) of brains from people who died with CTE. [Courtesy of Kondo et al., 2015. Nature.]
Clumps of tau, including its oligomers, aggregates, and tangles, show up in people who die years after traumatic brain injury (for a review, see Blennow et al., 2012). However, researchers rarely observe this pathology immediately after a severe blow to the head, making them question whether the tauopathy causes the neurodegeneration (Mannix et al., 2013; Smith et al., 2003).
Lu and colleagues previously reported that a prolyl isomerase called Pin1 transformed tau phosphorylated at threonine 231 from its toxic cis conformation to the physiological trans form (Liou et al., 2003). The team developed antibodies that could distinguish these cis/trans isomers, and reported that this cis version builds up in Alzheimer’s disease, where Pin1 activity falls (Nakamura et al., 2012). They wondered if cis p-tau formed after traumatic brain injury and if so, whether their antibody could halt any neurodegeneration that follows.
First authors Asami Kondo and Koorosh Shahpasand examined postmortem brain tissue from 16 people with CTE and a history of TBI and compared it to that of eight controls. Axons in CTE brains were riddled with cis p-tau—but not the trans form—and the protein co-localized with tau oligomers and tangles. Cis p-tau did not appear in dendrites, however, or in brains from healthy people.
To see if cis p-tau was an early consequence of TBI, the authors modeled brain injury from a blast or impact in mice. They saw that 12 hours after severe injury, cis, but not trans p-tau, rose in axons. Tau oligomers, aggregates, and tangles were not detectable. What’s more, the long-term trajectory of cis p-tau depended on the severity of injury. For instance, a single mild TBI bumped up levels initially, but they fell to normal after two weeks. A repeated mild TBI, or a single severe blow, on the other hand, sent levels soaring for weeks and months after injury.
Cis p-tau appeared to spread, as well. After repeat mild TBI, the protein stayed within the cortex for about two months, but after six turned up in other brain areas, such as the hippocampus. It appeared to be neurotoxic, because brain lysates from these 6-month-old mice killed cultured SY5Y neurons. They also disrupted the transport of mitochondria along microtubules. Treatment with the cis antibody prevented both.
Kondo and colleagues next wanted to see if this cis antibody might treat TBI. After a single severe TBI in wild-type mice, they treated the animals for two weeks with the monoclonal antibody for cis p-tau by injecting it once into the brain and into the periphery after that. This reversed the defective mitochondrial transport, disrupted long-term potentiation, and abundant apoptosis seen in TBI controls. Two months of treatment restored normal risk-taking behavior on the elevated-plus maze, a test that measures anxiety behavior. After six months on the antibody, the brains of these mice were devoid of TBI-associated tauopathy or atrophy in the cortex and white matter.
Why would TBI hike levels of cis p-tau in the first place? Kondo and colleagues found that brain damage downregulated Pin1 activity. They previously found that this happens when the death-associated protein kinase 1 (DAPK1) phosphorylates the enzyme and closes its active site (Lee et al., 2011). Lu predicted that brain injury activates various stress-related kinases, one of which disables Pin1, leading to the accumulation of cis p-tau.
“Based on our experiments, cis p-tau occurs long before later tau pathology,” said Lu. This suggests an antibody against the cis version could slow or halt the neurodegeneration that follows. He plans to humanize the cis antibody and test it in human patients with TBI. He will also examine whether cis p-tau could be a useful biomarker to identify TBI patients, or even those with AD, he said.
“It’s pretty clear based on the data that this cis p-tau is an early epitope in TBI,” said Einar Sigurdsson of the New York School of Medicine. He praised the thorough approach and suggested testing the cis antibody in models of other tauopathies. “It seems like a very promising treatment.” He guessed that other toxic tau epitopes arise at the same time as cis p-tau after injury. While he finds the data convincing, he did caution that the trans p-tau antibody they used had a significantly lower affinity for its target than the anti-cis p-tau antibody, which could influence the relative levels they reported.
Rakez Kayed, University of Texas Medical Branch, Galveston, agreed that the thorough set of experiments was technically sound. He predicted that cis p-tau is among the earliest tau isoforms to oligomerize, and could make a plausible therapeutic target. It may even appear in other tauopathies, he said. Interestingly, a recent study cataloging post-translational modification to tau, though not prolyl isomerization, found no difference between tau isoforms in normal mice and an APP-based transgenic model of AD. It did not look at changes in TBI (Jul 2015 news).
“The cis-antibody looks like a sensitive method for picking up TBI and early stages of tauopathy, making it a useful diagnostic tool,” wrote Stuart Rulten, University of Sussex, Brighton, U.K. “That they can block some of the neuropathological effects of TBI using this antibody demonstrates a potential therapeutic strategy for tauopathies in general,” he said.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012 Dec 6;76(5):886-99. PubMed.
- Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J, Zhang J, Bryant J, Rezaie S, Chung JY, Peters NV, Lee C, Tien LW, Kaplan DL, Feany M, Whalen M. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol. 2013 Jul;74(1):65-75. Epub 2013 Aug 6 PubMed.
- Smith C, Graham DI, Murray LS, Nicoll JA. Tau immunohistochemistry in acute brain injury. Neuropathol Appl Neurobiol. 2003 Oct;29(5):496-502. PubMed.
- Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003 Jul 31;424(6948):556-61. PubMed.
- Nakamura K, Zhou XZ, Lu KP. Distinct functions of cis and trans phosphorylated tau in Alzheimer's disease and their therapeutic implications. Curr Mol Med. 2012 Nov 15; PubMed.
- Lee TH, Chen CH, Suizu F, Huang P, Schiene-Fischer C, Daum S, Zhang YJ, Goate A, Chen RH, Zhou XZ, Lu KP. Death-associated protein kinase 1 phosphorylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell. 2011 Apr 22;42(2):147-59. Epub 2011 Apr 14 PubMed.
Further Reading
Papers
- Ahmed F, Plantman S, Cernak I, Agoston DV. The Temporal Pattern of Changes in Serum Biomarker Levels Reveals Complex and Dynamically Changing Pathologies after Exposure to a Single Low-Intensity Blast in Mice. Front Neurol. 2015;6:114. Epub 2015 Jun 12 PubMed.
- Washington PM, Villapol S, Burns MP. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy?. Exp Neurol. 2015 Jun 16; PubMed.
- Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP, Omalu B, Bailes J, Kepe V. In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):E2039-47. Epub 2015 Apr 6 PubMed.
- Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, Medcalf RL, Johnston LA, Hovens CM, Jones NC, O'Brien TJ. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain. 2015 May;138(Pt 5):1297-313. Epub 2015 Mar 13 PubMed.
News
- Brain Injury Boosts Dementia Risk
- For Hockey Players, Brain Damage Can Be Measured in Blood
- Does a Blow to the Head Mean More Amyloid Down the Road?
- Imaging Reveals Amyloid Up To a Year After Traumatic Brain Injury
- Meet the New Progressive Tauopathy: CTE in Athletes, Soldiers
- Blast Anatomy—Chronic Traumatic Encephalopathy in Military Vets
Primary Papers
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015 Jul 23;523(7561):431-6. Epub 2015 Jul 15 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.