Neurodegenerative Proteins in the Skin Could be Diagnostic
Quick Links
Neurodegenerative disease proteins hide behind the protective barriers of the central nervous system, complicating attempts to detect and track disease. While brain imaging and cerebrospinal fluid analyses detect some of these proteins, the techniques are complex, invasive, or expensive. Blood, though easy to obtain, has proven a poor source for neurodegenerative disease markers. What about the skin? At the annual meeting of the American Academy of Neurology, held April 18 to 25 in Washington, D.C., researchers reported that protein aggregates found in the brains of people with amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and Alzheimer’s disease (AD) appeared in the dermal layers, too. More research is needed to figure out what this could mean, but some think the skin could become a source of diagnostic markers.
In the developing embryo, brain and skin cells arise from the same ectodermal germ layer. Some scientists hypothesize, therefore, that some protein abnormalities found in neurons may also arise in the skin. In PD patients, deposits of α-synuclein were found in the nerves of the skin, though not in skin cells themselves (Doppler et al., 2014; Donadio et al., 2014; Wang et al., 2013). Other studies reported that skin changes can precede neurological problems in PD and ALS. For instance, people with PD had a greater incidence of melanoma, while some people with ALS were reported to have leathery, less-elastic skin that seemed to resist bedsores (Matsuo and Kamitani, 2010; Ono, 2000). It remains unclear if these conditions are related to the toxic proteins that accumulate in the brain, but some researchers are studying the question.
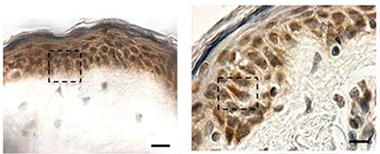
Hiding in Plain Sight:
Cytoplasmic TDP-43 inclusions (reddish brown) are absent in biopsied skin from healthy controls (left), but show up in patients with sporadic ALS (right). [Image courtesy of Paré et al., 2015.]
At AAN, Nicolas Dupré of Laval University, Québec, presented data from findings he and colleagues published last January. According to their study, TDP-43 forms cytoplasmic inclusions in fibroblasts from ALS patients (Paré et al., 2015). Senior author François Gros-Louis took arm skin biopsies from six people in each of three groups—healthy controls, people with sporadic ALS, and people who carried a hexanucleotide expansion in the C9ORF72 gene that causes ALS and frontotemporal dementia (five of whom were presymptomatic). They found TDP-43 aggregates in skin cells from all but the healthy controls (see image at left). Cytoplasmic TDP-43 inclusions are considered a hallmark of pathology; the protein normally resides in the nucleus (see Jan 2010 news story). Previously, researchers had reported elevated TDP-43 in the nuclei of skin cells from ALS patients (Suzuki et al., 2010).
Gros-Louis and colleagues did not stop there. They work closely with a lab that grows human-derived skin for burn victims, and hence decided to engineer the same kind of tissue from the biopsied material and see how it differed from normal skin. They isolated skin fibroblasts and grew them in individual layers. Then, placing one atop the other, they let them fuse. They added a layer of keratinocytes to form an epidermis. Two weeks later, the researchers had a three-dimensional model of human skin. Derived from healthy controls, the new skin developed a fully differentiated epidermis and neatly organized dermis. On the other hand, tissue grown from either C9ORF72 mutation carriers or sporadic ALS patients formed an undifferentiated epidermis with poorly fused layers (see image below). The structural skin protein collagen appeared disorganized, as well.

Model skin:
Tissue-engineered skin from healthy controls (left) has a well-developed dermis (blue) and epidermis (purple), unlike that from a C9ORF72 patient (right). [Image courtesy Paré et al., 2015.]
The TDP-43 antibody 12892-1-AP revealed the cytoplasmic aggregates of ALS pathology in almost a third of the cells of the synthetic skin derived from each patient. The cytoplasmic inclusions appeared both in tissue grown from symptomatic sporadic patients, and from pre-symptomatic C9ORF72 mutation carriers. By contrast, only 4 percent of cells in engineered skin from healthy donors had them. That the abnormalities cropped up in skin made from pre-symptomatic patients suggests that they may predict oncoming neurological disease. However, the researchers are unsure when these proteins accumulate in skin relative to the onset of neurological symptoms.
Because it can be cultured ad infinitum, the tissue-engineered skin could eventually offer a renewable source of human tissue to use in the search for reliable biomarkers, to test diagnostic methods, and perhaps to monitor the effectiveness of potential treatments, speculated Dupré, though more research is required.
Pathogenic proteins may appear in skin from AD and PD patients, as well, according to research led by Ildefonso Rodríguez-Leyva, Central Hospital, Ignacio Morones Prieto, San Luis Potosi, Mexico, and senior author María Jiménez-Capdeville, Universidad Autónoma de San Luis Potosi. At AAN, they reported recently published findings that α-synuclein and phosphorylated tau (p-tau) aggregate in the skin of people with PD, while p-tau also turns up in the skin of AD patients (Rodríguez-Leyva et al., 2015; Rodríguez-Leyva et al., 2014).
The researchers took small skin biopsies from behind the ears of 12 healthy controls, 16 people with PD, 17 with non-neurodegenerative dementias, and 20 with AD (diagnosed using NINCDS-ADRDA criteria; McKhann et al.,1984). The researchers tested the tissue with the antibodies AT8, which detects tau phosphorylated at serine 202 and threonine 205, and E178, which recognizes tau phosphorylated at serine 396, as well as non-phosphorylated tau. They also used RB-9026-P, a polyclonal antibody for α-synuclein. Immunohistochemistry and western blots indicated that cells from AD and PD patients bound seven and five times more AT8, respectively, than control cells. E178 detected no differences among the tissues, suggesting that total tau levels were unchanged. PD skin cells also had up to seven times more α-synuclein than control tissue.
Other scientists had previously observed phosphorylated tau in the brains of PD patients (Wills et al., 2010).
“These are preliminary findings and will have to be reproduced in larger groups of subjects with and without the diseases of interest,” said John Hart, University of Texas at Dallas, who co-chaired the symposium.
Jiménez-Capdeville will next check whether Aβ is present in patients’ skin cells, and whether the amount of any of these proteins increases over time in parallel with pathogenic processes in the brain. As with TDP-43, the scientists do not know when these proteins turn up in the skin relative to the onset of disease.
Skin cells could potentially offer a ready source of biomarkers for neurodegenerative diseases, said Tapan Khan, Blanchette Rockefeller Neurosciences Institute, Morgantown, West Virginia. Skin cells can be cultured and resampled. Khan is working on skin biomarkers for Alzheimer’s disease. He thinks cell signaling changes that precede protein aggregation in skin fibroblasts could catch disease earlier than waiting for those proteins to accumulate. His work suggests reduction in protein kinase Cε could herald AD in these cells (see Khan et al., 2015).
“The findings are interesting, but the authors have a lot more work to do to move these approaches further,” said John Trojanowski, University of Pennsylvania, Philadelphia. For starters, the authors need to confirm via biochemistry that the antibodies are truly picking up the neurodegenerative proteins in question. “The skin is notoriously fraught with artifacts because of non-specific binding of antibodies,” Trojanowski said. The sample sizes in these studies need to become larger, and subjects need autopsy confirmation of their underlying diseases, he said.—Gwyneth Dickey Zakaib
References
News Citations
Antibody Citations
Paper Citations
- Doppler K, Ebert S, Uçeyler N, Trenkwalder C, Ebentheuer J, Volkmann J, Sommer C. Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol. 2014 Jul;128(1):99-109. Epub 2014 May 1 PubMed.
- Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, Capellari S, Avoni P, Baruzzi A, Liguori R. Skin nerve α-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014 Apr 15;82(15):1362-9. Epub 2014 Mar 14 PubMed.
- Wang N, Gibbons CH, Lafo J, Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013 Oct 29;81(18):1604-10. PubMed.
- Matsuo Y, Kamitani T. Parkinson's disease-related protein, alpha-synuclein, in malignant melanoma. PLoS One. 2010;5(5):e10481. PubMed.
- Ono S. The skin in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Jun;1(3):191-9. PubMed.
- Paré B, Touzel-Deschênes L, Lamontagne R, Lamarre MS, Scott FD, Khuong HT, Dion PA, Bouchard JP, Gould P, Rouleau GA, Dupré N, Berthod F, Gros-Louis F. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol Commun. 2015 Jan 31;3:5. PubMed.
- Suzuki M, Mikami H, Watanabe T, Yamano T, Yamazaki T, Nomura M, Yasui K, Ishikawa H, Ono S. Increased expression of TDP-43 in the skin of amyotrophic lateral sclerosis. Acta Neurol Scand. 2010 Nov;122(5):367-72. PubMed.
- Ildefonso RL, Erika CA, Laura CG, Verónica MM, Martha S, et al. Presence of Phosphorylated Tau Protein in the Skin of Alzheimer´s Disease Patients. J Mol Biomark Diagn S6:005. doi: 10.4172/2155-9929.S6-005
- Rodríguez-Leyva I, Calderón-Garcidueñas AL, Jiménez-Capdeville ME, Rentería-Palomo AA, Hernandez-Rodriguez HG, Valdés-Rodríguez R, Fuentes-Ahumada C, Torres-Álvarez B, Sepúlveda-Saavedra J, Soto-Domínguez A, Santoyo ME, Rodriguez-Moreno JI, Castanedo-Cázares JP. α-Synuclein inclusions in the skin of Parkinson's disease and parkinsonism. Ann Clin Transl Neurol. 2014 Jul;1(7):471-8. PubMed.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939-44. PubMed.
- Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson's disease brains with and without dementia. Exp Neurol. 2010 Sep;225(1):210-8. PubMed.
- Khan TK, Sen A, Hongpaisan J, Lim CS, Nelson TJ, Alkon DL. PKCε deficits in Alzheimer's disease brains and skin fibroblasts. J Alzheimers Dis. 2015;43(2):491-509. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.